Chronic obstructive pulmonary disease
Adult: Maintenance treatment: As dry powder inhaler: 1 inhalation once daily. Max: 1 inhalation once daily.
|
Indications and Dosage
Oral
Chronic obstructive pulmonary disease Adult: Maintenance treatment: As dry powder inhaler: 1 inhalation once daily. Max: 1 inhalation once daily.
|
|
Special Precautions
Patient with severe CV disorders (especially cardiac arrhythmias); urinary retention, prostatic hyperplasia, bladder-neck obstruction; narrow-angle glaucoma. Not indicated for the rescue or initial treatment of acute episodes of bronchospasm or acutely deteriorating COPD. Not recommended in patients with asthma. Severe hepatic impairment. Pregnancy and lactation.
|
|
Adverse Reactions
Significant: CV effects (e.g. cardiac arrhythmia, atrial fibrillation, tachycardia), hypersensitivity reactions (e.g. anaphylaxis, angioedema, pruritus, rash, urticaria); increased intraocular pressure, exacerbated urinary retention.
Eye disorders: Blurred vision. Rarely, eye pain. Gastrointestinal disorders: Constipation, upper abdominal pain, toothache, dysgeusia, dry mouth. Musculoskeletal and connective tissue disorders: Arthralgia, myalgia. Nervous system disorders: Headache. Renal and urinary disorders: UTI, dysuria. Respiratory, thoracic and mediastinal disorders: Nasopharyngitis, upper respiratory tract infection, sinusitis, cough, pharyngitis. Potentially Fatal: Paradoxical bronchospasm. |
|
Inhalation/Respiratory: C
|
|
Monitoring Parameters
Monitor FEV1, peak flow, and other pulmonary function test prior to and periodically during treatment. Assess for signs and symptoms of urinary retention, glaucoma, and hypersensitivity reactions.
|
|
Overdosage
Symptoms: Visual accommodation disturbances, dry mouth, and tachycardia. Management: Symptomatic and supportive treatment. Discontinue therapy and appropriately monitor patient as necessary.
|
|
Drug Interactions
Additive effect with other anticholinergic drugs.
|
|
Action
Description: Umeclidinium bromide is a long-acting quaternary ammonium antimuscarinic or anticholinergic agent. It competitively and reversibly inhibits the binding of acetylcholine at type 3 muscarinic (M3) receptors in the bronchial smooth muscles, thereby causing bronchodilation.
Pharmacokinetics: Absorption: Rapidly absorbed from the lungs. Absolute bioavailability: Approx 13%. Time to peak plasma concentration: 5-15 minutes. Distribution: Volume of distribution: 86 L (IV). Plasma protein binding: Approx 89%. Metabolism: Metabolised in the liver mainly by CYP2D6 via oxidative (hydroxylation and O-dealkylation), followed by conjugation (glucuronidation) into a range of metabolites. It is a substrate for P-glycoprotein (P-gp) transporter. Excretion: Mainly via faeces (approx 58%); urine (approx 22%). Elimination half-life: Approx 11-19 hours. |
|
Chemical Structure
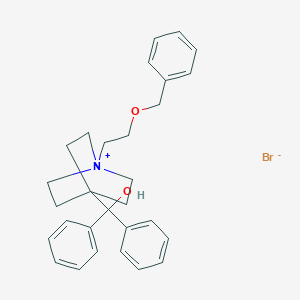 Source: National Center for Biotechnology Information. PubChem Database. Umeclidinium bromide, CID=11519069, https://pubchem.ncbi.nlm.nih.gov/compound/11519069#section=Structures (accessed on June 26, 2020) |
|
Storage
Store between 15-30°C. Protect from moisture, heat or sunlight.
|
|
MIMS Class
|
|
ATC Classification
R03BB07 - umeclidinium bromide ; Belongs to the class of other inhalants used in the treatment of obstructive airway diseases, anticholinergics.
|
|
References
Anon. Umeclidinium. Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 02/06/2020. Buckingham R (ed). Umeclidinium Bromide. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 02/06/2020. GlaxoSmithKline NZ Limited. Incruse Ellipta 62.5 micrograms, Powder for Inhalation data sheet 11 April 2017. Medsafe. http://www.medsafe.govt.nz/. Accessed 02/06/2020. Incruse Ellipta 55 micrograms Inhalation Powder, Pre-dispensed (GlaxoSmithKline [Ireland] Limited). European Medicines Agency [online]. Accessed 17/06/2020. Incruse Ellipta Aerosol Powder (GlaxoSmithKline LLC). DailyMed. Source: U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed/. Accessed 02/06/2020. Joint Formulary Committee. Umeclidinium. British National Formulary [online]. London. BMJ Group and Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 02/06/2020.
|